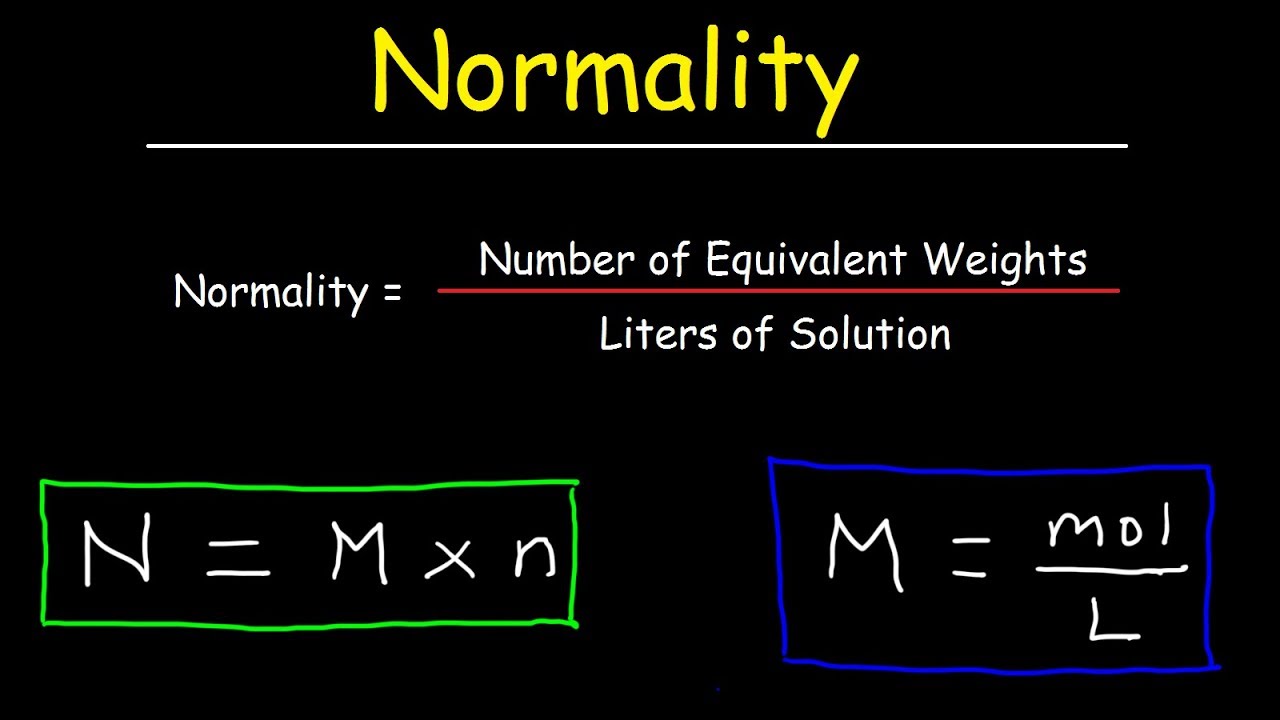
Acid Normality . normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. acid & base molarity & normality calculator.
from lessonschoolcatarrhous.z14.web.core.windows.net
normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. acid & base molarity & normality calculator. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions.
Acid Base Calculator Chemistry
Acid Normality acid & base molarity & normality calculator. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. acid & base molarity & normality calculator.
From byjus.com
If the Normality of acid and base is equal then what is the Normality Acid Normality normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base). Acid Normality.
From www.sigmaaldrich.cn
Acid and Base Chart — Table of Acids & Bases Acid Normality normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide. Acid Normality.
From www.youtube.com
Relationship between Normality and Molarity YouTube Acid Normality normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. acid & base molarity & normality calculator. it’s easy to calculate normality from molarity for an acid or base solution if you know. Acid Normality.
From studylib.net
NORMALITY Acid Normality normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. acid & base molarity & normality calculator. it’s easy to calculate normality from molarity for an acid or base solution if you know. Acid Normality.
From www.compoundchem.com
A Guide to Acids, Acid Strength, and Concentration Compound Interest Acid Normality normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. acid & base molarity & normality calculator. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality expresses concentration in terms of the. Acid Normality.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Acid Normality normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. it’s easy to calculate normality from. Acid Normality.
From www.numerade.com
SOLVED 'Determination the normality of a sodium hydroxide solution Acid Normality it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with. Acid Normality.
From ar.inspiredpencil.com
Normality Equation Acid Normality normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with. Acid Normality.
From www.numerade.com
SOLVED 100 ml of given KMnO4 solution titrates 10 ml of 0.1 m oxalic Acid Normality normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. acid & base molarity & normality calculator. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. it’s easy to calculate normality from molarity for an acid or base solution if you. Acid Normality.
From www.slideserve.com
PPT CHAPTER 16 (HOLT) ACIDBASE TITRATION AND pH PowerPoint Acid Normality normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with. Acid Normality.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID3842068 Acid Normality normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. it’s easy to calculate normality from. Acid Normality.
From bio.libretexts.org
3.1 Amino Acids and Peptides Biology LibreTexts Acid Normality normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. acid & base molarity & normality. Acid Normality.
From byjus.com
normality Acid Normality it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. acid & base molarity & normality calculator. normality expresses concentration in terms of the equivalents. Acid Normality.
From www.numerade.com
SOLVED CRB Normality of acids is also related to equivalents Acid Normality acid & base molarity & normality calculator. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. normality expresses concentration in terms of the equivalents. Acid Normality.
From www.pinterest.com
How to Calculate Normality of a Solution Chemistry lessons, Chemistry Acid Normality normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. acid & base molarity & normality. Acid Normality.
From byjus.com
The normality of 100 Acid Normality it’s easy to calculate normality from molarity for an acid or base solution if you know the number of hydrogen (acid) or hydroxide (base) ions. acid & base molarity & normality calculator. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. normality expresses concentration in terms of the. Acid Normality.
From brainly.in
normality of 2M sulphuric acid is....... explain a) 2N b) 4N c) N/2 d Acid Normality normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved per. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. acid & base molarity & normality calculator. it’s easy to calculate normality from molarity for an acid or base solution if you. Acid Normality.
From www.toppr.com
Normality of 2M sulphuric acid is Acid Normality acid & base molarity & normality calculator. normality expresses concentration in terms of the equivalents of one chemical species reacting stoichiometrically with another. normality expresses concentration in terms of the equivalents of one chemical species that react stoichiometrically with another. normality is defined as the number of equivalent weights (or simply equivalents, eq) of solute dissolved. Acid Normality.